Clinical Trials
Type 1 Diabetes Clinical Trials
Clicinal Trials
SDRI Conducts an average of 25 clinical trials annually and engages between 700 to 1,000 participants each year.
At SDRI, we are at the forefront of diabetes technology innovation. We have participated in key pivotal trials of commercial automated insulin delivery devices, and served as a clinical site in the trials for Medtronic’s closed-loop technology, Tandem Diabetes Control- IQ and Basal-IQ technology, and Insulet’s Automated Insulin Delivery System Omnipod 5.
SDRI has participated in key drug development clinical trials including Mannkind’s Afrezza inhaled insulin, Eli Lilly’s once-weekly basal insulin and GLP-1 inhibitors, Novo Nordisk’s fast acting insulin FiAsp and comparing Tresiba and Lantus over long haul travel, and a comparison between Sanofi’s drugs, Toujeo and Lantus.
Our dedicated clinical research team is making profound strides in developing and bringing new devices to market, significantly improving the lives of those living with diabetes.
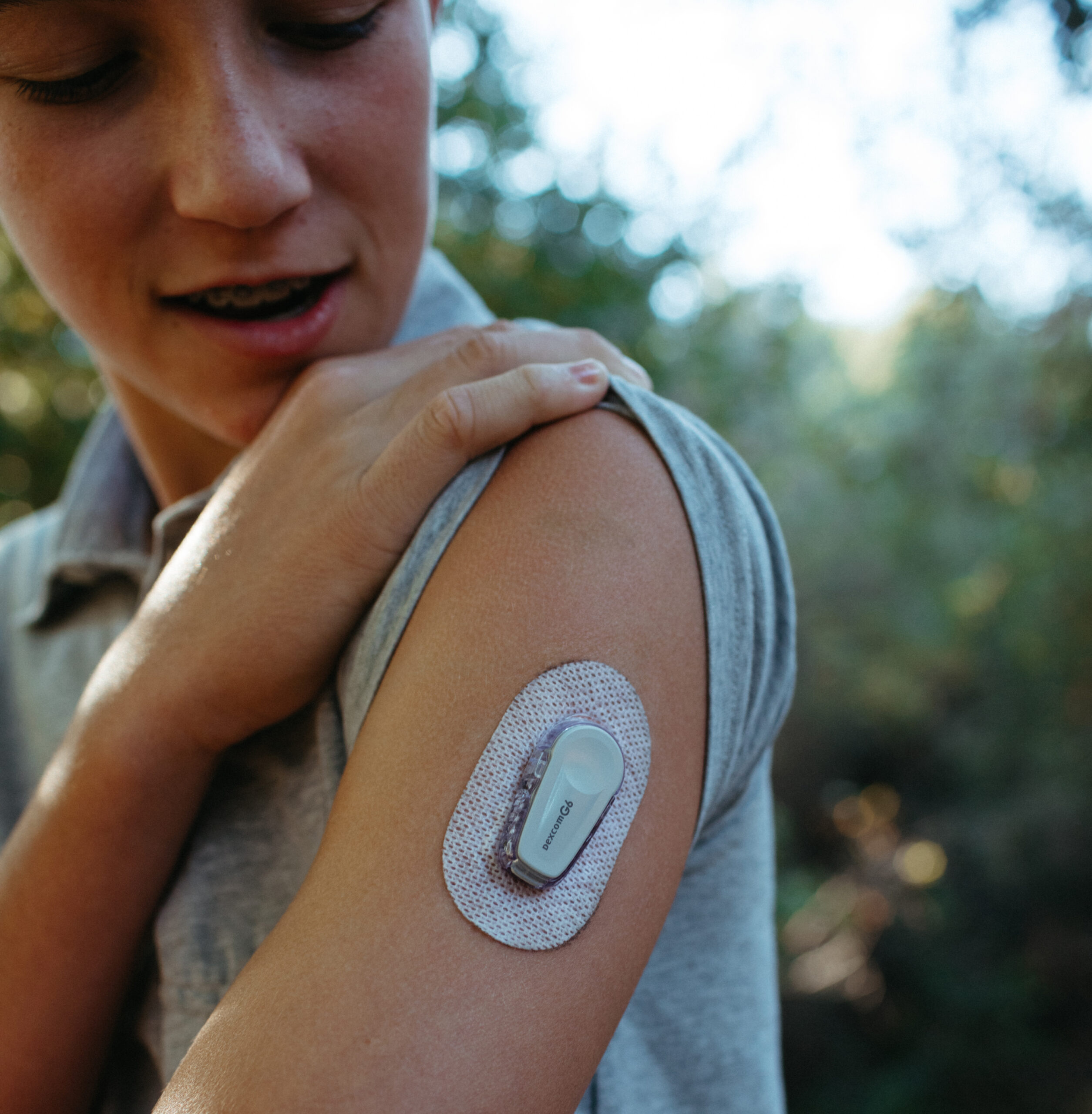

Your Participation
By participating in SDRI’s pediatric and adult clinical trials, participants and their families not only contribute to vital research but also gain access to cutting-edge treatments and technologies under the expert care of SDRI’s dedicated team. SDRI is committed to providing a supportive and compassionate environment for all participants, including Spanish translation services upoening up active participation for more people in our community.
“We are dedicated to reducing the burden of diabetes for all people, especially children. Our pediatric research programs offer families the opportunity to be part of the latest advancements in diabetes science, contributing to innovations that will shape the future of diabetes care and hopefully reduce the burden, so kids can think about being an astronaut or race car driver and not let diabetes get in the way of their dreams.”
– Dr. Kristin Castorino
SDRI’s Vice President of Clinical Research and Senior Investigator
Opportunities
Want To Learn More?
Contact
Temporary Location
5425 Hollister Ave, Suite 230
Santa Barbara, CA 93111
Phone: 805-682-7638
Fax: 805-682-3332
Patient Care: 805-682-4793
Tax ID # 95-1684086



